Our groups research a wide variety of self-assembling and active biopolymer systems. These systems are made from particles of biological origin and thus exhibit the unmatched complexity and reproducibility seen in nature. Studying these biological ensembles enables us to uncover novel phenomena that are not easily observed in conventional chemical systems.
When compared to most rods grown via chemical synthesis, filamentous viruses have a very high degree of monodispersity, i. e. all the viruses have the same length. Due to this quality, we use filamentous viruses to study a large range of phenomena that are not usually observed in comparable synthetic systems. In addition, we can systematically mutate virus DNA to vary its contour length, flexibility and chirality. This gives us control over important microscopic parameters and establishes a firm experimental connection between microscopic parameters and macroscopic behavior.



Microtubules are very stiff polar filaments assembled from monomeric tubulin proteins. We study the mechanical properties of these polymers with a home-made optical tweezing system. Microtubules also form bundles in the presence of a depleting agent such as PEG. These bundles can exhibit motile activity when mixed with kinesin motor clusters and ATP, thus forming isotropically active gels. At high enough densities, active microtubule bundles take on the properties of liquid crystals in the nematic phase. As bundles continue to exhibit activity in this phase, we call this system an active nematic.
Bacteria spin filaments with helical shape (bacterial flagella) at very high speed in order to propel themselves forward. Flagella have unique polymorphic transitions between several states with widely different helicities. Entire flagella can switch between two states in response to a variety of external stimuli (pH, ionic strength, temperature ...). Bacterial flagella are unique in that they allow us to investigate how filament helicity influences macroscopic assembly and behavior.
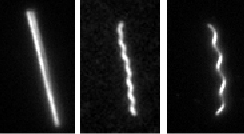
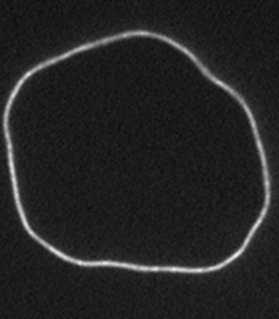
F-Actin is a biologically important filament that assembles from the monomeric protein G-Actin. It is a semiflexible polymer with a persistence length of 16 microns. If purified correctly, the contour length of actin filaments can be larger then 100 microns. Their large size and persistence length make actin filaments ideally suited for direct visualization and manipulation with optical microscopy. Although most actin filaments are linear, we have also defined conditions which greatly enhance the assembly of actin filaments with ring conformation. Moreover, we have shown that certain fluorescent labels induce actin filaments to switch between multiple conformations.